Abstract
Background: Ivosidenib (IVO) and enasidenib (ENA) are oral inhibitors of mutant IDH1 (mIDH1) and mutant IDH2 (mIDH2), respectively, FDA-approved for the treatment of relapsed/refractory IDH-mutant acute myeloid leukemia (AML). Here we report updated response and survival results from a phase 1 study of these agents when combined with intensive chemotherapy in patients with newly diagnosed m IDH1/2 AML.
Methods: The design of this open-label, multicenter, phase 1 study (NCT02632708) has been previously described. Briefly, eligible patients with newly diagnosed m IDH1 or m IDH2 AML were treated with induction therapy (daunorubicin 60 mg/m 2/day or idarubicin 12 mg/m 2/day × 3 days with cytarabine 200 mg/m 2/day × 7 days) in combination with either IVO 500 mg once daily (for m IDH1) or ENA 100 mg once daily (for m IDH2). After induction, patients received up to 4 cycles of consolidation therapy while continuing the mIDH inhibitor. Patients who completed or were ineligible for consolidation continued on maintenance IVO or ENA until the end of the study. IDH mutation clearance and measurable residual disease (MRD) negativity were assessed using BEAMing digital PCR and multiparameter flow cytometry (Stein et al. Blood 2021).
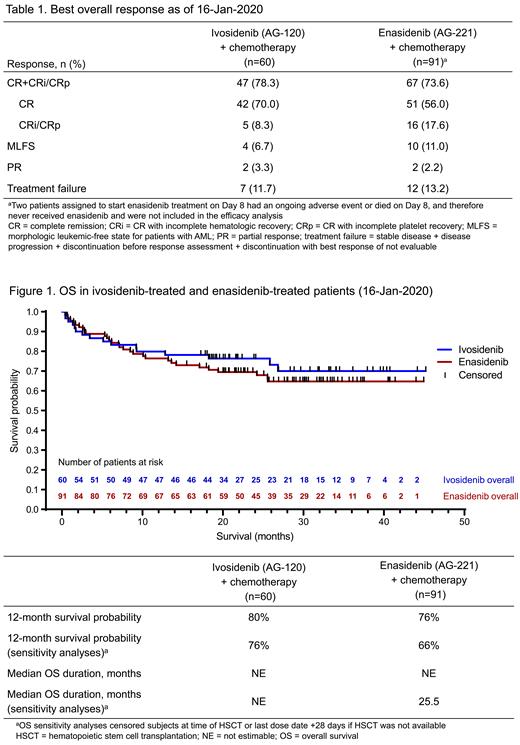
Results: As of 16-Jan-2020, 153 patients had been treated: 60 in the IVO cohort (median age 62.5 years, range 24-76) and 93 in the ENA cohort (median age 63.0 years, range 27-77); 2 patients assigned to start ENA on Day 8 had an ongoing adverse event or died on Day 8, and therefore never received ENA and were not included in the efficacy analysis. Secondary AML (sAML; arising after an antecedent hematologic disorder, or after exposure to genotoxic injury) was present in 18/60 (30.0%) IVO-treated patients and in 35/93 (37.6%) ENA-treated patients. In patients with sAML, 4 (22.2%) and 17 (48.6%) IVO-treated and ENA-treated patients, respectively, had previously received a hypomethylating agent. IVO or ENA combined with induction and consolidation were well tolerated (Stein et al . Blood 2021). Among the 60 IVO-treated patients, a response of complete remission (CR), CR with incomplete hematologic recovery (CRi), or CR with incomplete platelet recovery (CRp) was achieved in 37/42 (88.1%) patients with de novo AML and in 10/18 (55.6%) patients with sAML. Among the 91 ENA-treated patients, a response of CR, CRi, or CRp was achieved in 45/56 (80.4%) patients with de novo AML and in 22/35 (62.9%) patients with sAML. Best overall response is reported in Table 1. Patients achieving CR, CRi, or CRp who had available samples were analyzed for IDH mutation clearance and MRD negativity. In those treated with IVO, the IDH1 mutation was cleared in 16/41 (39.0%) patients, and 16/20 (80.0%) were considered MRD negative. In those treated with ENA, the IDH2 mutation was cleared in 15/64 (23.4%) patients, and 10/16 (62.5%) were MRD negative (Stein et al . Blood 2021). Thirty-five (58.3%) IVO-treated patients received ≥1 cycle of consolidation therapy, 18 (30.0%) patients received maintenance after consolidation, 1 (1.7%) patient received maintenance after induction, and 29 (48.3%) patients proceeded to hematopoietic stem cell transplantation (HSCT). Forty-six (49.5%) ENA-treated patients received ≥1 cycle of consolidation therapy, 17 (18.3%) patients received maintenance after consolidation, 7 (7.5%) patients entered maintenance without consolidation, and 43 (46.2%) patients proceeded to HSCT.
Median durations of follow-up were 21.2 and 23.7 months for IVO and ENA, respectively. For patients who entered maintenance, median duration of active maintenance was 13.8 and 11.0 months for IVO and ENA, respectively. Of patients who achieved CR, 7/42 (16.7%) of those treated with IVO and 7/51 (13.7%) of those treated with ENA experienced relapse or death. Overall survival is reported in Figure 1. Updated data from July 2021 will be presented.
Conclusion: IVO or ENA in combination with induction and consolidation therapy have shown acceptable safety profiles, with ≥80% CR+CRi/CRp remission rates in patients with m IDH de novo AML. With over 21 months of follow-up, overall survival rates were high, with 12-month survival probabilities of >75% for both the IVO- and ENA-treated patients. The clinical benefit of adding IVO or ENA to induction, consolidation, and maintenance therapy for patients with newly diagnosed m IDH AML is being further evaluated in the ongoing HOVON150AML randomized phase 3 trial (NCT03839771).
Stein: Jazz Pharmaceuticals: Consultancy; Foghorn Therapeutics: Consultancy; Blueprint Medicines: Consultancy; Gilead Sciences, Inc.: Consultancy; Abbvie: Consultancy; Janssen Pharmaceuticals: Consultancy; Genentech: Consultancy; Bristol Myers Squibb: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Astellas: Consultancy; Syndax Pharmaceuticals: Consultancy; Syros Pharmaceuticals, Inc.: Consultancy; Agios Pharmaceuticals, Inc: Consultancy; PinotBio: Consultancy; Daiichi Sankyo: Consultancy. DiNardo: Takeda: Honoraria; Novartis: Honoraria; AbbVie: Consultancy, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Forma: Honoraria, Research Funding. Fathi: Kite: Consultancy, Honoraria; Foghorn: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Trillium: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Blueprint: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; Servier: Research Funding; Agios: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Morphosys: Consultancy, Honoraria; Ipsen: Consultancy, Honoraria. Mims: Syndax Pharmaceuticals: Consultancy; Abbvie: Consultancy; Genentech: Consultancy; Kura Oncology: Consultancy; Leukemia and Lymphoma Society: Consultancy; BMS: Consultancy; Jazz Pharmaceuticals: Consultancy; Aptevo: Research Funding; Glycomemetics: Research Funding; Xencor: Research Funding; Daiichi-Saynko: Consultancy. Savona: Karyopharm: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS-Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ryvu: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Taiho: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ALX Oncology: Research Funding; Astex: Research Funding; Incyte: Research Funding. Stein: Amgen: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Stemline: Speakers Bureau. Stone: Boston Pharmaceuticals: Consultancy; Elevate Bio: Membership on an entity's Board of Directors or advisory committees; BerGen Bio: Membership on an entity's Board of Directors or advisory committees; Innate: Consultancy; Janssen: Consultancy; Jazz: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy, Research Funding; Onconova: Consultancy; Syndax: Membership on an entity's Board of Directors or advisory committees; Syntrix/ACI: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Foghorn Therapeutics: Consultancy; Astellas: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Arog: Consultancy, Research Funding; Gemoab: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy; Aprea: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Actinium: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Agios: Consultancy, Research Funding; Celgene: Consultancy; Macrogenics: Consultancy. Winer: Abbvie: Consultancy; Takeda: Consultancy; Novartis: Consultancy. Döhner: AstraZeneca: Consultancy, Honoraria; Astex: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Berlin-Chemie: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria; Pfizer: Research Funding; Ulm University Hospital: Current Employment; Roche: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; GEMoaB: Consultancy, Honoraria; Helsinn: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Oxford Biomedicals: Consultancy, Honoraria. Pollyea: Syndax: Honoraria; Takeda: Honoraria; Astellas: Honoraria; Karyopharm: Consultancy, Honoraria; Foghorn: Honoraria; Kiadis: Honoraria; Syros: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Teva: Research Funding; Amgen: Honoraria; Aprea: Honoraria; Jazz: Honoraria; Gilead: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Celgene: Honoraria; Bristol Myers Squibb: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding. McCloskey: Jazz: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Pfizer: Consultancy; Novartis: Consultancy; Amgen: Speakers Bureau; Incyte: Speakers Bureau; COTA: Other: Equity Ownership; BMS: Honoraria, Speakers Bureau. Odenike: AbbVie, Celgene, Impact Biomedicines, Novartis, Taiho Oncology, Takeda: Consultancy; Celgene, Incyte, AstraZeneca, Astex, NS Pharma, AbbVie, Gilead, Janssen, Oncotherapy, Agios, CTI/Baxalta, Aprea: Research Funding. Ossenkoppele: Agios: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Abbvie, AGIOS, BMS/Celgene Astellas,AMGEN, Gilead,Servier,JAZZ,Servier Novartis: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Astellas: Consultancy, Honoraria. Patel: Aptevo Therapeutics: Research Funding; BMS-Celgene, Agios: Membership on an entity's Board of Directors or advisory committees; Peerview: Honoraria. Roshal: Celgene: Other: Provision of services; Auron Therapeutics: Other: Ownership / Equity interests; Provision of services; Physicians' Education Resource: Other: Provision of services. Frattini: Celgene/BMS: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Cellectis, Inc.: Current Employment, Current equity holder in publicly-traded company. Lersch: Celgene, a Bristol-Myers Squibb Company: Current Employment. Nabhan: Agios: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Almon: Agios: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Saatcioglu: Servier Pharmaceuticals: Current Employment. Zhang: Servier: Current Employment; Agios: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Cooper: Agios: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months; Servier: Current Employment. Kantarjian: Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; AbbVie: Honoraria, Research Funding; Aptitude Health: Honoraria; Ipsen Pharmaceuticals: Honoraria; Astellas Health: Honoraria; Amgen: Honoraria, Research Funding; Ascentage: Research Funding; BMS: Research Funding; Astra Zeneca: Honoraria; Novartis: Honoraria, Research Funding; Jazz: Research Funding; Immunogen: Research Funding; KAHR Medical Ltd: Honoraria; NOVA Research: Honoraria; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Tallman: Kura: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; NYU Grand Rounds: Honoraria; Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Biosight: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Oncolyze: Membership on an entity's Board of Directors or advisory committees; KAHR: Membership on an entity's Board of Directors or advisory committees; Orsenix: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Rafael Pharmaceuticals: Research Funding; Glycomimetics: Research Funding; Biosight: Research Funding; Orsenix: Research Funding; Abbvie: Research Funding; Mayo Clinic: Honoraria; UC DAVIS: Honoraria; Northwell Grand Rounds: Honoraria; NYU Grand Rounds: Honoraria; Danbury Hospital Tumor Board: Honoraria; Acute Leukemia Forum: Honoraria; Miami Leukemia Symposium: Honoraria; New Orleans Cancer Symposium: Honoraria; ASH: Honoraria; NCCN: Honoraria.
Ivosidenib and enasidenib are indicated for the treatment of adult patients with relapsed or refractory AML with a susceptible IDH1 mutation (ivosidenib) or an IDH2 mutation (enasidenib) as detected by an FDA-approved test. Enasidenib and ivosidenib are investigational products in tumors other than relapsed/refractory AML.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal